- Call Today +90 537 762 59 24
- Open Hour Open 24 Hours
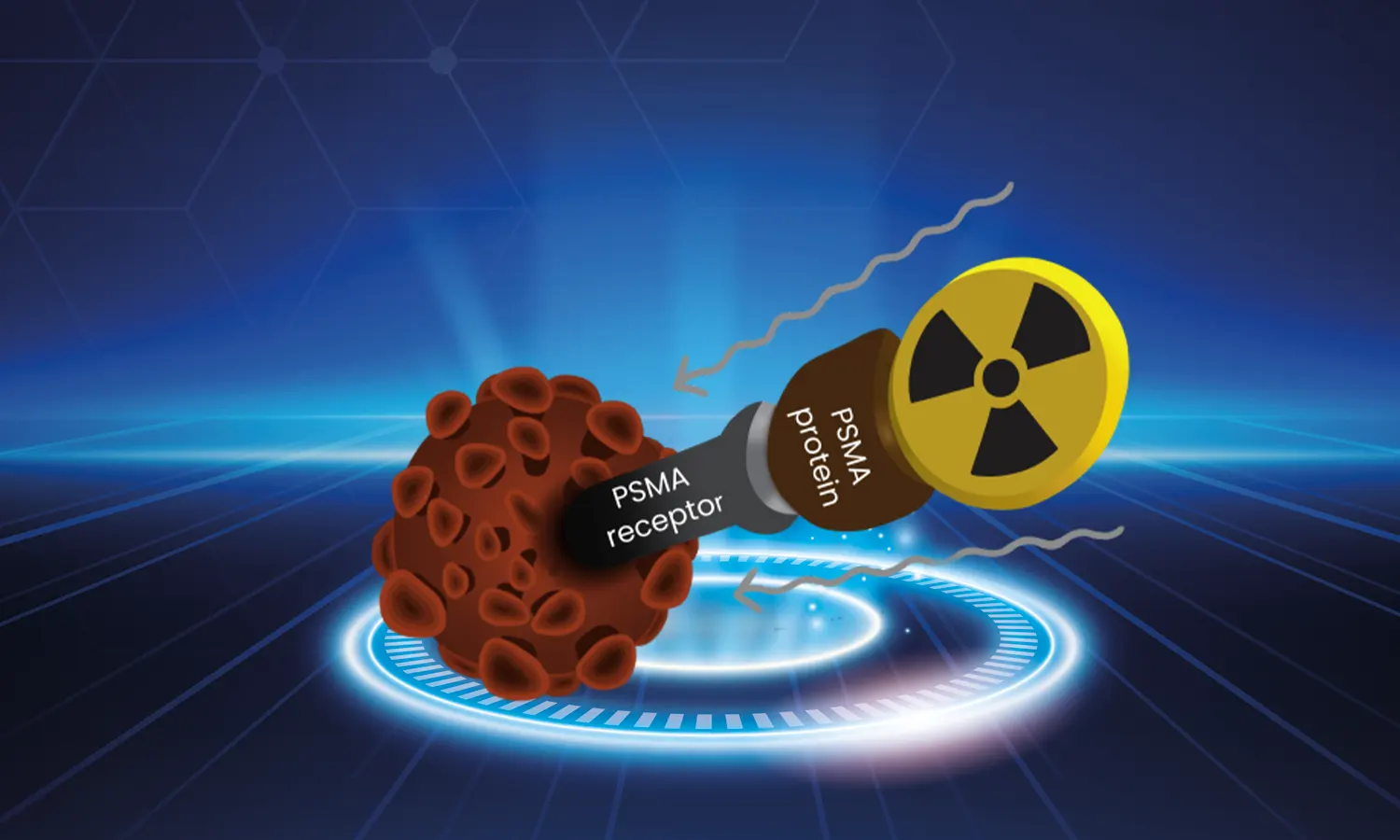
Lutetium 177 (Lu-177) is a radioactive isotope utilized in the treatment of specific cancers. Its primary application lies in combating prostate cancer that has metastasized to other regions of the body. This treatment involves the infusion of a compound named PSMA-617, incorporating Lu-177, directly into the patient's bloodstream. PSMA-617 is meticulously crafted to target prostate-specific membrane antigen (PSMA), a protein prevalent on the surface of prostate cancer cells. Upon administration, Lu-177 emits radiation, effectively exterminating cancerous cells.
Lutetium 177 PSMA treatment is typically reserved for patients grappling with metastatic prostate cancer, particularly in scenarios where conventional therapies like chemotherapy or hormone treatment have faltered or when the cancer relapses following prior treatment.
It's imperative to acknowledge that Lutetium 177 PSMA treatment isn't universally applicable. Certain medical conditions such as renal or hepatic impairments, along with pregnancy or breastfeeding in women, might preclude its usage. Additionally, individuals allergic to the treatment compounds are ineligible for this therapy.
The advantages of Lutetium 177 PSMA treatment encompass its potent efficacy in targeting metastatic prostate cancer cells, its minimal side effect profile owing to Lu-177's short half-life, and its adaptability in administration methods, tailored to individual patient needs. Furthermore, some studies hint at an improved quality of life post-treatment due to its outpatient nature and reduced side effects.
Common side effects of Lutetium 177 PSMA treatment include transient nausea, vomiting, diarrhea, fatigue, skin irritation at the injection site, and temporary declines in blood cell counts, predisposing patients to infections, bruising, and bleeding. However, these effects are typically fleeting and self-resolving.
Lutetium 177 PSMA treatment operates by delivering PSMA-617, housing Lu-177, into the bloodstream, targeting PSMA-presenting cancer cells. Upon infusion, Lu-177 emits beta particles, inducing cancer cell death by damaging their genetic material. Typically administered on an outpatient basis, the treatment duration and frequency hinge on cancer stage and patient-specific factors.
Primarily, Lutetium 177 PSMA treatment is employed against metastatic prostate cancer resistant to conventional therapies.
Before initiating treatment, patients undergo a test involving PSMA-11 injection, containing a minute amount of Lu-177, to assess suitability. Dosage frequency varies based on individual cancer progression and medical history, typically administered outpatient.
Lutetium 177 PSMA treatment is typically delivered intravenously, allowing patients to return home the same day. Preceding infusion, the injection site is prepared, anesthesia may be applied, and the compound is slowly administered via needle and syringe into the arm vein.
Following infusion, patients are advised to rest briefly before discharge, with instructions for self-care and subsequent monitoring appointments.
© 2026 Copyright by Private Koru Hospital. All rights reserved.
